A close look into the initial step of primer synthesis by an archaeoeukaryotic primase
(together with Prof. Allain, ETHZ and Prof. Wiegand RWTH Aachen)
The Sulfolobus islandicus multi-copy plasmid pRN1 encodes a particular replication protein endowed with both primase and helicase activities. The primase domain of the replication protein is of the archaeoeukaryotic type and consists of the highly conserved catalytic domain (Prim_Pol) and an accessory helix-bundle-domain (HBD). The HBD is essential for primase activity3. Our structural and functional investigations illustrate the HBD’s remarkable ability to selectively bind single-stranded DNA and two nucleotides, despite its relatively compact size (~110 amino acids). Employing nucleotides analogues, the primase reaction leading to the dinucleotide (the first product of primer synthesis) could be slowed down considerably. This allowed us to follow this critical reaction involving the catalytic domain and the HBD by NMR spectroscopy (Wu et al., 2024).
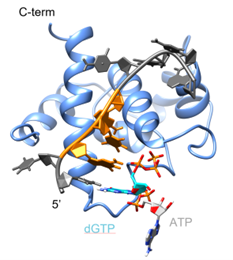
Wu, P., Zehnder, J., Schröder, N., Blümmel, P.E.W., Salmon, L., Damberger, Fred.F., Lipps, G., Allain, F.H.-T., Wiegand, T., 2024. Initial Primer Synthesis of a DNA Primase Monitored by Real-Time NMR Spectroscopy. J. Am. Chem. Soc. 146, 9583–9596. https://doi.org/10.1021/jacs.3c11836