Biosynthesis Rescue
Synthetic glycolipids for the treatment of rare diseases
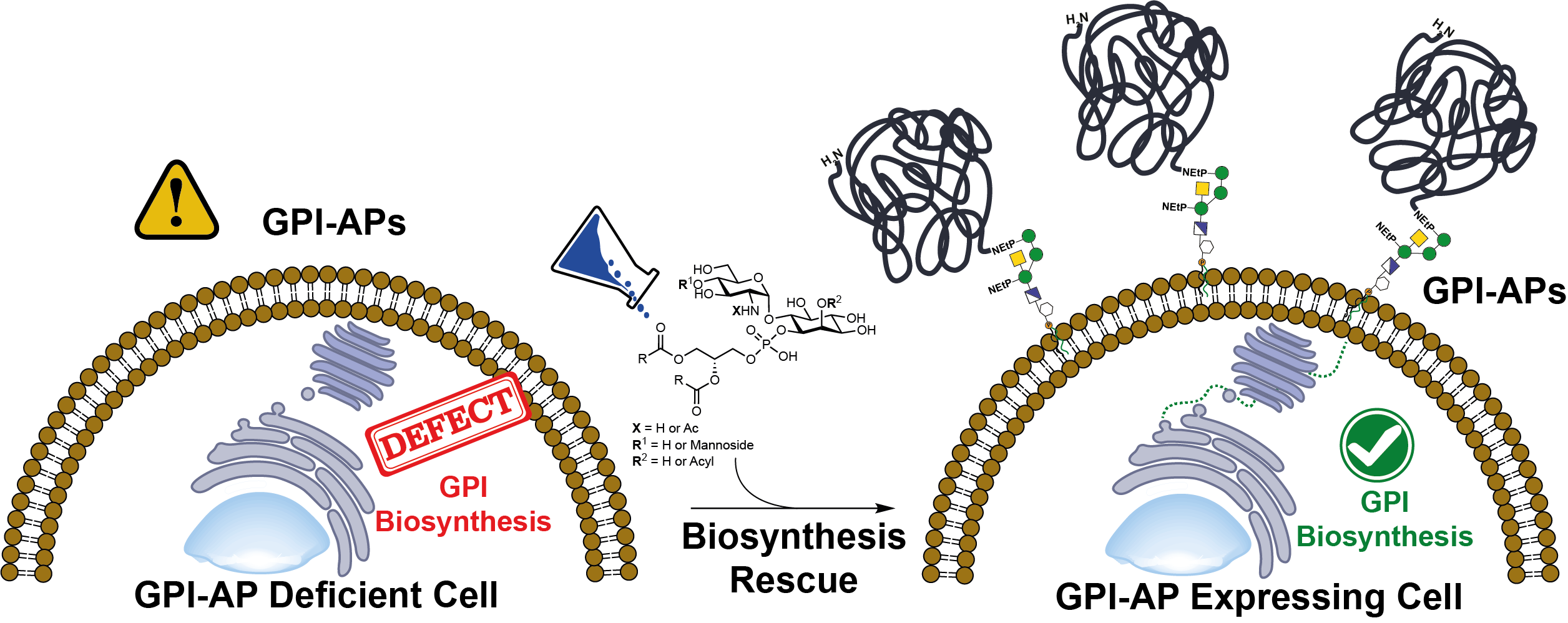
We established a synthetic strategy for the chemical synthesis of glycosylphosphatidylinositols having unsaturated lipids. This strategy was used in a project with the group of Prof. Taroh Kinoshita from Osaka University and the Max Planck Institute of Colloids and Interfaces to investigate the biosynthesis of GPI-anchored proteins (GPI-APs). We designed, synthesized, and used GPI fragments to rescue the biosynthesis of GPI-APs caused by a mutation in genes involved in assembling GPI-glycolipids in cells. We demonstrated that the synthetic fragments GlcNAc-PI, Man-GlcN-PI, and GlcN-PI with two and three lipid chains rescue the deletion of the GPI biosynthesis in cells devoid of the PIGA, PIGL, and PIGW genes in vitro. The compounds allowed for concentration-dependent recovery of GPI biosynthesis and were highly active on the cytoplasmic face of the endoplasmic reticulum membrane. These synthetic molecules lead to the development of treatments for IGDs and tools to study GPI-AP biosynthesis.
Applications
Deficiencies in the biosynthesis of GPIs and the concomitant production of GPI-anchored proteins lead to a series of rare and complicated disorders associated with inherited GPI deficiencies (IGDs) in humans. Currently, there is no treatment for patients suffering from IGDs. Studies performed with the Kinoshita Group from Osaka University demonstrated the applicability of synthetic glycolipids to rescue the GPI-biosynthesis in vitro in cells having knock-out genes and a dependency on the structure in the site of activity. Current studies are running to determine the mechanism of the glycolipids insertion into the process and the applicability of the compounds to treat rare diseases derived from GPI-deficiencies in animal models. The project is sponsored by the FHNW – Thanks a lot!
The initial results of the project and further applications of the glycolipids are reported in the following articles:
P. Guerrero, Y. Murakami, A. Malik, P. Seeberger, T. Kinoshita, and D. Varon Silva, „Rescue of Glycosylphosphatidylinositol-Anchored Protein Biosynthesis Using Synthetic Glycosylphosphatidylinositol Oligosaccharides, ACS Chem. Biol. 2021, Bd. 16, Nr. 11, S. 2297–2306, doi: 10.1021/acschembio.1c00465.
Wang Y. A. K. Menon; Y. Maki; Y.-S. Liu; Y. Iwasaki; M. Fujita; P. Guerrero; D. Varon Silva; P. Seeberger; Y. Murakami and T. Kinoshita, Genome-wide CRISPR screen reveals CLPTM1L as a lipid scramblase required for efficient glycosylphosphatidylinositol biosynthesis “, Proc. Natl. Acad. Sci. 2022, 119, 14, e2115083119, doi: 10.1073/pnas.2115083119
Comments
No comment posted about Biosynthesis Rescue