Cleanroom - Sterile Pharmaceutical Production
Pharmaceuticals must be produced in cleanrooms to ensure that they contain no impurities, especially if they are to be administered as infusions or injections.

In the PTC cleanroom, the entire process chain for various sterile dosage forms can be reproduced on a pilot scale. This includes all critical steps from compounding, filling, lyophilization and terminal sterilization to the visual inspection of the product. This wide scope makes the PTC cleanroom particularly suitable for developing processes with scale-up/scale-down potential, filling reference samples, manufacturing drug product for preclinical studies or process development for sterile pharmaceutical production on a pilot scale under non-GMP conditions.
The PTC facility is equipped with class C and D cleanrooms for processing chemical and biological drugs and for production of sterile solutions, emulsions and suspensions. Up to 10 litres of product solution can be filled automatically or manually into prefilled syringes (format 1ml long, staked needle) or vials (format 6R). Subsequently, up to 3000 vials per batch can be lyophilized.
Practical training in the PTC cleanroom allows students to learn about and experience sterile pharmaceutical production processes and cleanroom qualification and operation. Specialists will also be able to use the cleanroom for further training in the future: A PDA/FHNW course "Aseptic Processing" is planned.

Infrastructure
- Class C and D cleanrooms (EU GMP guideline)
- Formulation compounding in temperature-controlled glass or stainless steel containers (0.5-10 l)
- Cleanroom washing machine, capacity 500 l
- Steam sterilization, capacity 305 l
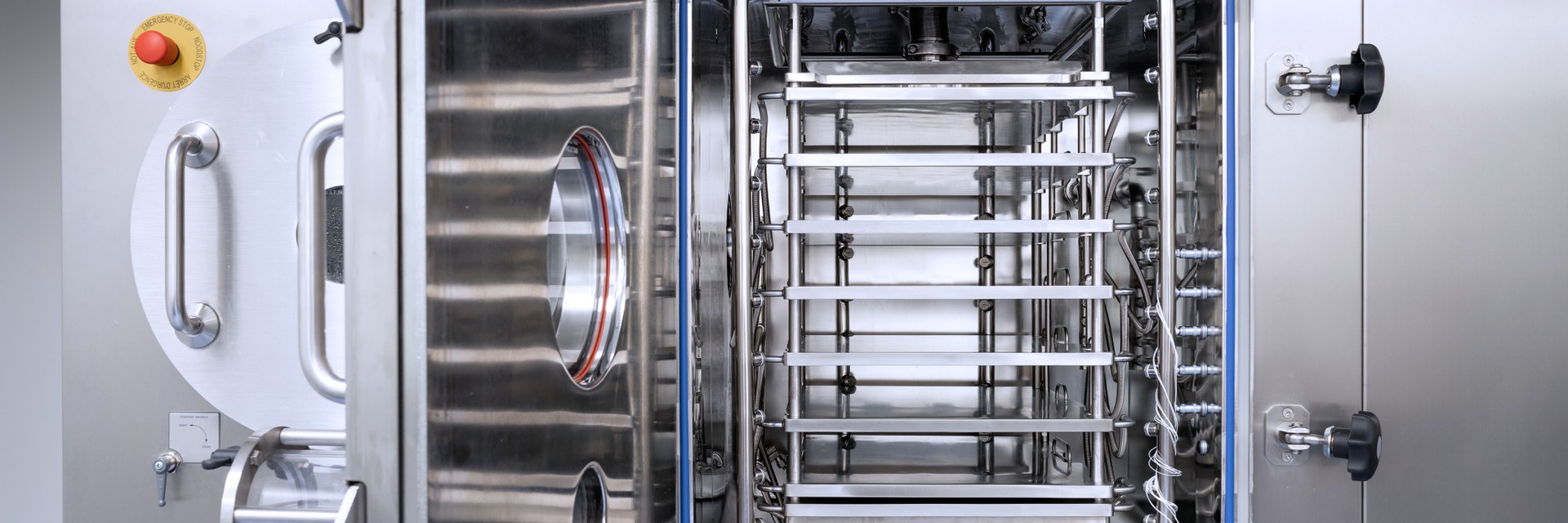
- Freeze dryer 2.02 m2 floor space
- Automatic filling line, peristaltic pump, nested packaging, up to 1000 units/hour
- Heat sterilization/de-pyrogenisation up to 260°C, capacity 450 l
- Safety workbench class 2, ISO 5
- Mobile cleanroom monitoring (particles, air velocity, temperature, relative humidity)
- Climate cabinets for stability studies including photostability according to ICH
- Controlled storage of active ingredients and products at -60 °C, -20 °C and 2-8 °C
